About CeNTech
The Center for Nanotechnology (CeNTech) provides the infrastructure for direct and interdisciplinary collaborations involving faculties of the University of Münster (chemistry, physics, biology and medicine). Research at CeNTech keeps a strong relation to technological applications and shall result in patents for new nanotechnology based inventions which can be developed up to the product level.
more
Latest News

Novermber 8, 2023 - Events
CeNTech Science Breakfast
Once again the traditional CeNTech Science Breakfast provided insights into current research activities within the science park and a valuable exchange of the stakeholders. The program included presentations on the production of natural rubber from dandelions, performed by Prof. Dirk Prüfer, innovative approaches in battery electrode research (Cabot Corporation), the application of simulated Raman microscopy in histology (Refined Laser Systems GmbH), the hydrogen cluster Münster and the activities of the SFB 1459 "intelligent matter".

First German-Dutch MedTech networking workshop in Münster
The first German-Dutch cross border MedTech networking event took place at the IHK Nord Westfalen. The workshop was organized by CeNTech and Bioanalytik Münster e.V. with the support of the Wirtschaftsförderung Münster GmbH. Healthcare professionals from the University Medical Center Muenster talked about clinic challenges/needs and upcoming project ideas for obvious existing medical/clinical needs. As the current challenges are similar in both regions - Münster-Land and the Twente region - companies and healthcare professionals discussed how to connect both regions in a better way and how to learn from each other. Furthermore the optimization of the transfer of tech innovation into clinics was addressed.
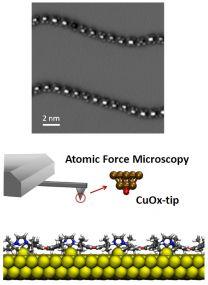
NATURE Publication: Polymers of ballbot-type carbenes
A team of scientists from the departments of chemistry and physics at the WWU Münster and researchers from Beijing used on-surface synthesis under extremely clean and defined conditions to realize the polymerization of N-heterocyclic carbenes (NHCs). The NHC units of these polymers are each connected to single gold atoms, which drastically increases their mobility on the different substrates (similar to a ballbot, i.e. a robot moving on a sphere). The detailed properties of the polymers were investigated by ultrahigh-resolution atomic force microscopy in the CeNTech labs. The study was recently published in Nature Chemistry (2023).


